Discovery of the link between CD63 tetraspanin and cholesterol transport
Discovery of the link between CD63 tetraspanin and cholesterol transport
The ICAN Omics Lipidomics (Dr Marie Lhomme) and ICAN I/O (Dr Maharajah Ponnaiah) platforms contributed to a recent scientific publication published on June 17, 2024 in Nature Cell Biology, a high-impact journal, along with the Dr. Anatol Kontush (UMRS 1166 – IHU ICAN).
Research has focused on the role of tetraspanin CD63, an important component of extracellular vesicles, in intercellular cholesterol transport.
Extracellular vesicles (such as exosomes) are recognized as key players in intercellular communication, and their role is influenced by certain proteins and lipids enriched when they are generated as intraluminal vesicles in multivesicular endosomes.

Results of the research project
- CD63 tetraspanin has been shown to transport cholesterol to intraluminal vesicles in cells, generating a pool that can be mobilized by the NPC1/2 complex, and exported via exosomes to recipient cells.
- In the absence of CD63, cholesterol is extracted from endosomes by actin-dependent vesicular transport.
- CD63 and cholesterol are therefore at the heart of a balance between internal and external endomembrane budding.
- These results therefore establish that CD63 tetraspanin is a key element in endosomal lipid transport, and show that intraluminal vesicles and exosomes constitute an alternative pathway for cellular cholesterol transport.

Epicardial adipose tissue discovery: better prevention of atrial metabolic cardiomyopathy with MRI
Epicardial adipose tissue discovery: better prevention of atrial metabolic cardiomyopathy with MRI
IHU ICAN research teams have successfully demonstrated the feasibility of isolating and measuring pure epicardial adipose tissue (TAE) from the atrioventricular grooves using routine cardiovascular magnetic resonance imaging (MRI), in order to better assess atrial cardiomyopathy and perhaps ultimately identify the risk of atrial fibrillation earlier.
Les résultats ont été publiés le 14 juin 2024 par European Heart Journal – Imaging Methods and Practicdans la publication scientifique “The Adipose Tissue Confined in the Atrio-Ventricular Groove can be used to Assess Atrial Epicardial Adipose Tissue and Atrial Dysfunction during Cardiac Magnetic Resonance Imaging”.
Epicardial adipose tissue (EAT): a biomarker of atrial fibrillation (AF) that is difficult to isolate
Epicardial adipose tissue (EAT) is attracting growing interest in medical research, to better understand and treat cardiovascular diseases, and more broadly cardiometabolic diseases.
- The TAE is an ectopic adipose tissue in contact with the myocardium (heart muscle), which surrounds the coronary arteries.
- In particular, it is used as a biomarker for atrial fibrillation (AF), the 1st cardiac cause of vascular embolic accidents (strokes), leading to significant morbidity and mortality in the population.
However, the use of epicardial adipose tissue as a biomarker in this setting remains limited, as it is difficult to isolate from other paracardiac adipose tissues.
Teams at IHU ICAN have therefore conducted a research project to test the feasibility and value of measuring pure epicardial adipose tissue contained in the atrioventricular groove (GEAT), using cardiovascular magnetic resonance imaging (MRI), in patients with distinct metabolic disorders.
Method and results of the research project
- The ICAN Imaging platform performed cardiovascular MRI on 100 patients from the MetaCardis cohort (INSERM), with criteria for obesity, metabolic syndrome and type 2 diabetes, and on age- and sex-matched healthy controls.
- A strong correlation between atrioventricular groove adipose tissue (AVSAT) volume and atrial epicardial adipose tissue (EAT) was obtained. AVSAT volume was higher in the 3 groups of patients with metabolic disorders and allowed them to be identified from controls after adjustment for body mass.
- Thanks to the logistic regression and machine learning methods used by the ICAN I/O platform, an algorithm has also been created to identify patients with type 2 diabetes.
The results suggest that atrioventricular groove adipose tissue, measured during routine MRI examination, can serve as a surrogate for atrial TEA, and be integrated into a multiparametric imaging biomarker to improve the assessment and early detection of atrial cardiomyopathy in diabetic patients.
This innovative strategy also opens up new avenues for early identification of the risk of atrial fibrillation, particularly in patients with type 2 diabetes, obesity and metabolic syndrome.
The actors involved in the project
- Jonathan Bialobroda, Intern in Cardiology, Pitié-Salpêtrière Hospital, AP-HP, Sorbonne University
- Khaoula Bouazizi,Operational manager of the ICAN Imaging platform, IHU ICAN, Research engineer, Biomedical Imaging Laboratory (LIB), Inserm
- Maharajah Ponnaiah,ICAN Data I/O Platform Manager, IHU ICAN
- Nadjia Kachenoura,Team leader, Biomedical Imaging Laboratory (LIB), Sorbonne University, Inserm, CNRS
- Etienne Charpentier, Cardiovascular imaging radiologist, Hôpital Pitié-Salpêtrière, AP-HP, Sorbonne University
- Mohamed Zarai, Medical imaging engineer (MRI and CT-Scan), Software developer, IHU ICAN
- Pr Karine Clement, Team leader Nutriomics unit, PU-PH, Nutrition Department, Hôpital de la Pitié-Salpêtrière, AP-HP, IHU ICAN
- Pr Fabrizio Andreelli, Nutriomics Unit, Diabetology and Metabolism Department, Hôpital de la Pitié-Salpêtrière, AP-HP, IHU ICAN
- Pr Judith Aron-Wisnewsky, Nutriomics Unit, Nutrition Department, Hôpital de la Pitié-Salpêtrière, AP-HP, IHU ICAN
- Pr Stéphane Hatem,Director of ICAN, Cardiologist, Hôpital de la Pitié-Salpêtrière, AP-HP
- Pr Alban Redheuil, Head of ICAN Imaging, IHU ICAN, PU-PH, Institut de Cardiologie, Hôpital de la Pitié-Salpêtrière, AP-HP, Laboratoire d’Imagerie Biomédicale (LIB), Sorbonne Université, Inserm, CNRS
Results of the MAESTRO-NASH clinical trial: a new hope for the treatment of MASH (NASH) with liver fibrosis
Results of the MAESTRO-NASH clinical trial: hope for the treatment of MASH (NASH) with liver fibrosis?
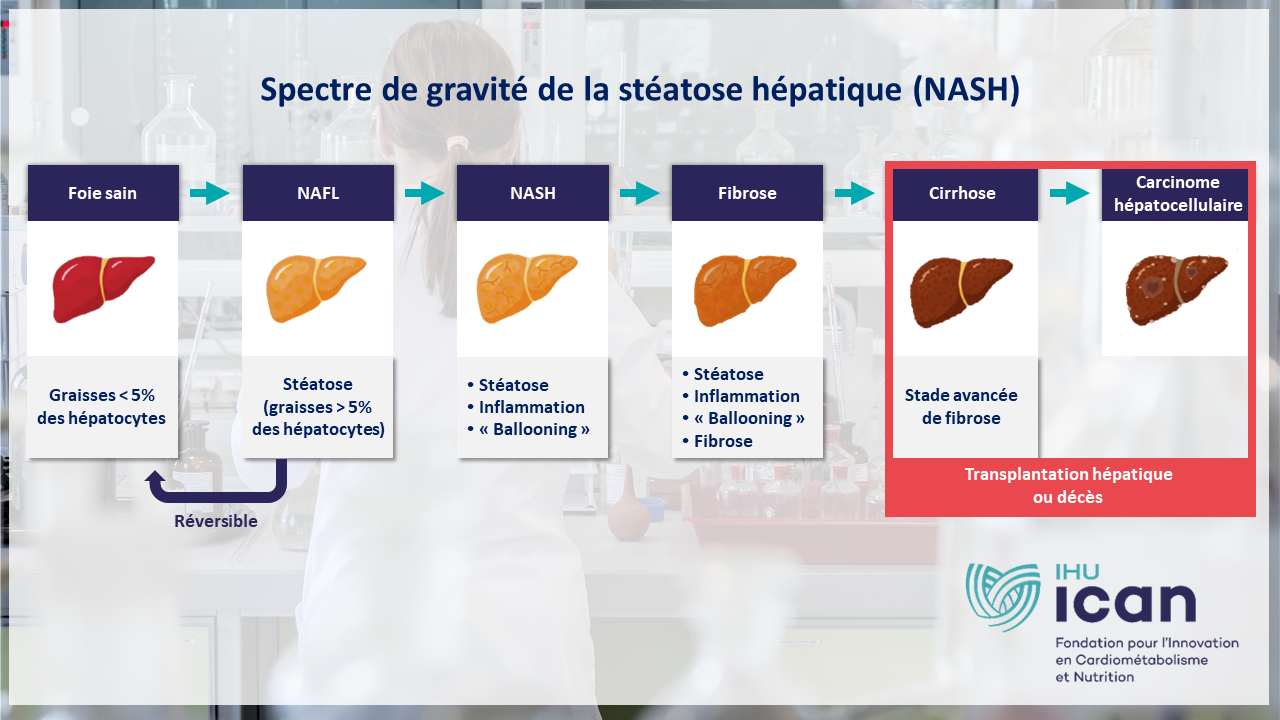
Metabolic steatohepatitis or MASH (formerly NASH) is a progressive liver disease for which there is no approved treatment. Yet “fatty liver disease” affects 25% of the world’s population, and it is estimated that 1 in 5 patients with MAFLD (hepatic steatosis associated with a metabolic disorder) will develop MASH.
Resmetirom is an orally administered, liver-targeted, beta-selective agonist of the thyroid hormone receptor. This treatment, currently under development, is used in the treatment of MASH accompanied by liver fibrosis (affecting around 1.5% of the general population).

Prof. Vlad Ratziu (Hepatologist, AP-HP, Sorbonne University, Inserm, IHU ICAN) has participated as co-lead investigator in a new Phase III clinical trial on the efficacy of resmetirom in the treatment of MASH.
The results were reported on February 8, 2024 in the prestigious scientific journal The New England Journal of Medicine: “A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis”.
On March 14, 2024, the FDA (Food and Drug Administration) approved resmetirom as the 1st drug to treat metabolic steatohepatitis (MASH)!
The methodology: a phase 3 clinical trial in patients with MASH (NASH) and fibrosis
- Resolution of MASH (NASH), without worsening fibrosis, was achieved in:
- 25.9% of patients in the 80 mg resmetirom group,
- 29.9% of those in the 100 mg resmetirom group,
- compared with 9.7% in the placebo group.
- Improvement of fibrosis by at least one stage, without worsening of the NAFLD activity score, was achieved in:
- 24.2% of patients in the 80 mg resmetirom group,
- 25.9% of those in the 100 mg resmetirom group,
- compared with 14.2% of those in the placebo group.
- The change in low-density lipoprotein cholesterol from baseline to week 24 was :
- -13.6% in the group receiving 80 mg resmetirom
- -16.3% in the 100 mg resmetirom group,
- compared with 0.1% in the placebo group.
- The incidence of serious adverse events was similar in all trial groups:
- 10.9% in the resmetirom 80 mg group,
- 12.7% in the resmetirom 100 mg group,
- 11.5% in the placebo group.
- Diarrhea and nausea were more frequent with resmetirom than with placebo.
The 80 mg and 100 mg doses of resmetirom were superior to placebo in resolving NASH and improving liver fibrosis by at least one stage.
A breakthrough in the drug treatment of MASH (NASH)
This trial opens up new prospects for the creation of a treatment to combat non-alcoholic steatohepatitis, or MASH, the prevalence of which is increasing year after year throughout the world.
In fact, this cardiometabolic disease can lead to other serious pathologies, due to the metabolic dysfunctions observed in patients:
- Depending on the presence of risk factors, the prevalence of NAFLD varies from 5.2% in non-diabetic subjects of normal weight, to 90% in diabetic and obese subjects.
- Among patients with NAFLD, the prevalence of advanced fibrosis is estimated at 2.5%, corresponding to around 230,000 people in mainland France.
- These figures are reinforced by the epidemic of obesity and type 2 diabetes in France and around the world.
IHU ICAN experts are developing cutting-edge research projects to build tomorrow’s medicine for cardiometabolic diseases.
A specialist in metabolic steatohepatitis (MASH), Prof. Vlad Ratziu has taken part in numerous therapeutic trials on this pathology, and has published over 300 articles in numerous international journals. He works at the ICAN IHU’s NASH clinic, which offers an innovative, personalized care pathway to optimize the diagnosis and management of non-alcoholic fatty liver.
A new diagnostic tool to improve MASH (NASH) clinical trials
A new diagnostic tool to improve clinical trials on MASH (NASH), or “fatty liver disease”.
In clinical drug trials, liver biopsy is now the standard examination required to diagnose metabolic steatohepatitis or MASH (formerly known as NASH). This condition, also known as “fatty liver disease”, is a liver disease that affects over 18% of the French population.
However, liver biopsy is invasive and often feared by patients, making it difficult to carry out therapeutic trials.
Prof. Vlad Ratziu, hepatologist and renowned teacher-researcher in the treatment of MASH, has taken part in a research project aimed at proposing alternative solutionsthrough the use of a blood test called NIS2+™.

Discover below the key findings of its scientific publication “NIS2+TM as a screening tool to optimize patient selection in metabolic dysfunction-associated steatohepatitis clinical trials”, published on December 5, 2023 in the Journal of Hepatology.
What is MASH or hepatic steatosis?
Today, 18.2%* of the French population are affected by hepatic steatosis (MASH – formerly NASH), a liver disease caused by an accumulation of fat of metabolic origin, independent of alcohol consumption or viral hepatitis*.(source: INSERM Constances cohort). This figure could rise to 24% by 2030.
This cardiometabolic disease is very often the consequence of an excessively sedentary lifestyle, combined with a diet too rich in fats and sugars. Le risque est une évolution vers des maladies graves telles que la cirrhose ou le cancer du foie.
People with MASH are often asymptomatic and at greater risk of developing cardiovascular disease, hypertension or diabetes.
The method: using the NIS2+™ test in diagnosis
A better selection of patients prior to liver biopsy would reduce the failure rate in clinical trials.
Standard fibrosis biomarkers (FIB)-4 do not detect progressive forms of metabolic steatohepatitis (MASH).
The work carried out by GENFIT, in collaboration with IHU ICAN, therefore compared the performance of screening incorporating the NIS2+™ test, based on a blood test, against current practices which involve biopsying patients simply on the basis of unspecific clinical information.
This NIS2+™ test was developed by the French biotechnology company GENFIT, based on a large collaborative international database. It allows to reduce the number of biopsies by better targeting patients likely to have the disease of interest, and to reduce the cost of carrying out these tests.
A retrospective simulation analysis was conducted in the diagnostic pathway of the RESOLVE-IT cohort, integrating the calculation and comparison of the following elements:
- Liver biopsy failure rate,
- Number of patients required for screening,
- Estimates of the overall cost of the various courses,
- Analysis of potential recruitment bias based on histology, gender, age or comorbidities.
The results: better use of liver biopsies
This study demonstrated that the use of the NIS2+™ test in selecting patients for liver biopsy significantly reduced unnecessary biopsies and screening costs, which could greatly improve the feasibility of clinical trials to combat MASH.
The cohort analyzed included 1,929 patients, 40% with at-risk MASH. The use of NIS2+™ significantly reduced the liver biopsy failure rate (39%) compared with non-specific biomarkers (58%) or the clinical diagnostic pathway alone (60%). This is accompanied by a significant reduction in associated costs.
The NIS2+™ test is an accurate and reliable screening tool that could improve patient recruitment in future MASH clinical trials, and increase comfort and safety for patients, while optimizing trial execution and cost-effectiveness.

IHU ICAN’s NASH (MASH) clinic

To improve diagnosis and earlier management of MASH (NASH), teams from AP-HP and IHU ICAN have set up the NASH clinic in 2019, headed by Pr Vlad Ratziu and Dr Raluca Pais.
This integrated care pathway aims to optimize diagnosis, provide patients with information (therapeutic education) and initiate a comprehensive care for people with hepatic steatosisThis includes diagnosis of all associated diseases (type 2 diabetes, hypertension, dyslipidemia, risk of heart attack or stroke).
The ultimate aim is to slow down, or even halt, the progression of MASH towards serious forms (cirrhosis, liver cancer), requiring major interventions such as liver transplantation.
MEDITWIN: using digital twins to develop tomorrow's personalized medicine
MEDITWIN: using digital twins to develop tomorrow’s personalized medicine
How can the use of virtual twins improve the quality of care, making healthcare safer and more accessible for all? This is the mission of MEDITWIN, a consortium of 7 Instituts Hospitalo-Universitaires (IHUs) including IHU ICAN, from Nantes University Hospital, Inria, associated startups and Dassault Systèmes, announced on Monday, December 11, 2023 in the presence of French President Emmanuel Macron..
Thanks to personalized virtual twins of organs, metabolism and cancerous tumors, this innovative project will make it possible to diagnose the risk of cardiovascular disease for high-risk patients, and to help specialized physicians choose treatments for effective patient follow-up.
The MEDITWIN initiative will be developed over 5 years, from 2024 to 2029. The partners’ investment in this project will be financially supported by the French government as part of France 2030.


What medical need does MEDITWIN meet?
“Digital twins open up the prospect of a new form of precision and preventive medicine for cardiometabolic diseases, by establishing for each individual his or her future risk of developing cardiovascular disease, based on risk factors such as his or her genomic signature. Such a project requires a change of scale in research, which has now been achieved thanks to the project led by Dassault Systèmes, with all the MEDITWIN partners on board.”
Prof. Stéphane Hatem, General Director of the ICAN IHU and Director of UMR 1166 Cardiovascular and Metabolic Diseases

Cardiometabolic diseases, also known as cardiovascular-metabolic diseases, refer to a group of interconnected disorders affecting the heart and metabolic system. They mainly include: cardiovascular diseases affecting the heart and blood vessels (coronary heart disease, hypertension…) and metabolic disorders (type 2 diabetes, obesity, hypercholesterolemia, steatotic liver disease – MASH-(NASH)).
Cardiovascular disease is the world’s leading cause of death, accounting for almost 18 million deaths every year. In France, they represent the 2nd cause of death in the general population, with almost 150,000 deaths each year, and the 1st cause in women. More than 15 million people are treated in France for disease, cardiovascular risk or diabetes, at an annual cost of over 17 billion euros.
To date, all symptomatic and asymptomatic patients at risk are assessed for cardiovascular risk by their physicians.
The association between certain classic biological markers (classic lipid profile, LDL-cholesterol) and the risk of cardiovascular disease is indisputable. However, to date, the use of these markers in medical practice does not allow for fine stratification of this population.
Without predictive tools, it is difficult to individualize patient follow-up and choose the most appropriate treatments and optimal doses, while reducing the side effects associated with treatment (statins).
Technical advances not yet systematized
Scientific and biomedical research has made significant advances in this field, with plans to integrate other markers based on imaging and Omics data (metagenomics, metabolomics, lipidomics, proteomics).
Advances in imaging techniques, such as thoracic CT scans (large and small arteries), now enable non-invasive, detailed analysis of vascular damage in patients with no known cardiovascular disease.
By revealing the progression, stabilization or regression of the disease and predicting the quality of response to treatment, these new indicators become a concrete means of applying prevention and targeted therapies according to the patient’s profile and biology.

Familial hypercholesterolemia (FH) is a major cause of premature cardiovascular disease, and represents a model of accelerated pathological progression. Following the trajectory of these patients is therefore an opportunity to access risk prediction indicators within a limited timeframe.
Recent data have shown that, even in the case of familial hypercholesterolemia, the use of non-invasive coronary imaging can predict the incidence of cardiovascular events in asymptomatic subjects, with high predictive power over a follow-up of less than 3 years.
However, these techniques are not fully integrated into decision-making algorithms, and their use in clinical practice is not systematized.
Using virtual twins for a better treatment strategy
With this use case for virtual twins, the MEDITWIN consortium’s ambition is to develop a personalized predisposition diagnostic service for cardiovascular disease, and to select the best treatment strategies, while integrating the patient’s environment, such as gut microbiota and nutrition.
The predictions of such twins in terms of trajectories and their improvement could support the practitioner in the management of the patient, and also make more tangible the patient’s future in the absence of intervention in order to improve adherence to treatment. This will make it possible to measure the impact of proposed solutions in terms of reducing the number of patients with cardiovascular events (coronary, cerebrovascular, arterial…).
The benefits will be felt by patients, who will benefit from care tailored to their individual risk and directly linked to the progress of their disease, and by practitioners, who will be able to take advantage of tools for the early identification and management of chronic diseases.
MEDITWIN will therefore enable a digital twin model to be established to monitor the individualized cardiovascular risk of patients with familial hypercholesterolemia, thanks to a selection of relevant biomarkers.
Digital twins will help to clarify the cause-and-effect relationship between micro and macro-circulation in a healthy cardiovascular system, so that we can better understand the pathophysiological mechanisms of aging, hypertension, diabetes and other conditions.
Clarivate 2023 ranking: 2 ICAN researchers among the most cited in the world!
Clarivate 2023 ranking: 2 ICAN researchers among the most cited in the world!
Clarivate’s “Highly Cited Researchers” ranking highlights the most influential researchers worldwide, with highly cited scientific publications over the last decade. It includes the top 1% of the world’s most cited researchers.
Our warmest congratulations go to Prof. Vlad Ratziu and Prof. Alain Combes, members of the ICAN IHU community, who feature in this 2023 ranking!


-
Prof. Vlad Ratziu (Gastroenterology and hepatology)
Prof. Ratziu’s research focuses on metabolic liver diseases, mainly metabolic steatosis (fatty liver).
He has taken part in therapeutic trials on metabolic steatohepatitis conducted with the ICAN IHU and AP-HP, and has published over 300 articles in numerous international journals.
Prof. Ratziu coordinated the Fatty Liver Inhibition of Progression (FLIP) consortium, which aimed to study both the mechanisms of liver disease progression in metabolic steatosis and therapeutic interventions, based on one of the largest patient cohorts in Europe.
He is co-editor of the Journal of Hepatology, the official journal of the European Association of Liver Studies (EASL).
-
Prof. Alain Combes (Intensive care medicine)
Prof. Alain Combes’ research focuses on the care of critically ill cardiac patients, mechanical circulatory assistance and extracorporeal membrane oxygenation, rescue therapies for severe respiratory failure, and infections in critically ill patients.
Professor Combes is a member of the European Society of Intensive Care Medicine (ESICM), the American Thoracic Society (ATS), the European Society of Cardiology (ESC), the Board of Directors of the Association for Acute Cardiovascular Care (ESC-ACVC), the Extracorporeal Life Support Organization (ELSO), where he is former Chairman of EURO-ELSO, the International ECMO Network (ECMONet) and the French Resuscitation Society (SRLF).
Prof. Combes is Deputy Editor-in-Chief of Intensive Care Medicine.

IHU ICAN launches its barometer on cardiometabolic diseases with IFOP
Cardio-metabolic diseases: we’re all concerned!
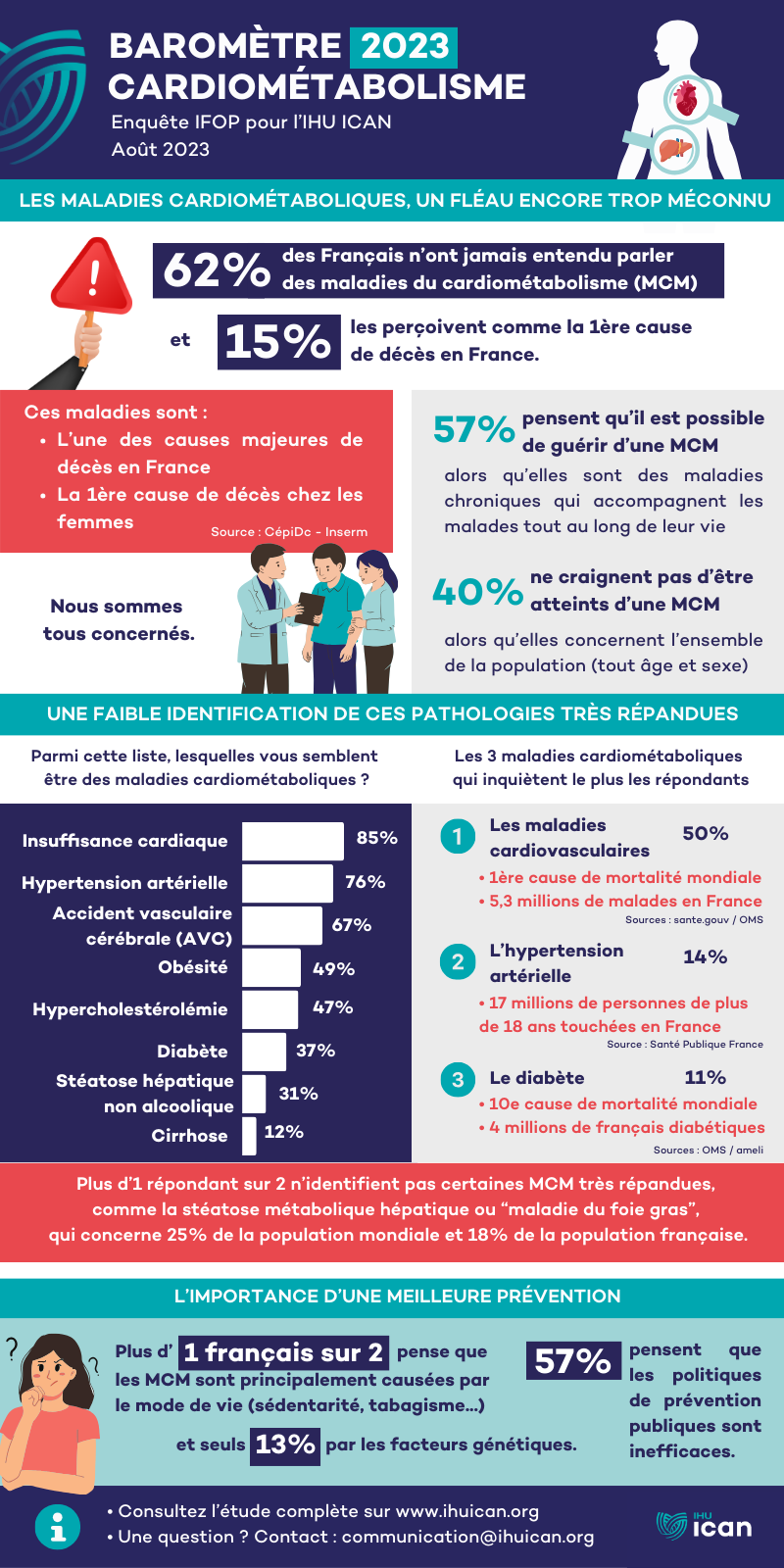
To better inform and raise public awareness of one of the major causes of death in France, the IHU ICAN is launching its cardiometabolic barometer (IFOP / IHU ICAN study – September 2023), drawing on its extensive expertise in research and the fight against cardiometabolic diseases.
- What do the French know about cardiometabolism?
- How do they view these pathologies?
- Are they informed about causes and risk factors?

A veritable public health scourge, cardiometabolic diseases have been steadily on the rise in recent years. Today, they represent a major cause of death in France and the 1st cause of death worldwide (source: CépiDc/Inserm).
They include many diseases such as diabetes, obesity, cardiovascular disease (hypertension, thrombosis, cardiomyopathy, stroke, heart failure), liver disease (including hepatic metabolic steatosis or fatty liver disease), and hypercholesterolemia.
How these chronic diseases work is still too little known to the general public, as cardiometabolism is an emerging discipline that requires a high level of expertise. cutting-edge scientific and medical research to understand these complex, interconnected pathologies, linked to an imbalance in the individual’s metabolism and/or to genetic factors. They are very common and often diagnosed too late, as they develop silently in the body.
Yet cardiometabolic diseases concern us all.
A few figures (in France):
- Cardiovascular diseases: 5.3 million sufferers, more than 140,000 deaths per year
- Diabetes: 4 million people treated, 35,000 diabetes-related deaths
- Obesity: 17% of adults are obese, 47% overweight
- Hepatic metabolic steatosis (NASH): 18% of the population affected, almost 24% in 2030 (IHU ICAN estimate)
Sources : ameli, Santé publique France
Cardiometabolic diseases, a scourge still too little known
The first lesson to be learned from this study, cardiometabolic diseases are poorly identified by the French: only 38% have heard of them and 10% see exactly what it’s all about. Young women and donors to medical research – two audiences traditionally more aware of health issues – are slightly more likely to declare that they know about pathologies (45% and 55%).
The 38% of French people who say they know about these diseases have only a partial knowledge of them. If they rightly identify heart failure (85%), hypertension (76%) and stroke (67%) as diseases of the cardiometabolic system, they are less than 1 in 2 know that obesity (49%), hypercholesterolemia (47%) and diabetes (37%) also belong to this family of diseases. Non-alcoholic hepatic steatosis (20%) and cirrhosis (12%) are even less closely related to this family.
As a corollary to this poor understanding of cardiometabolic diseases, their contribution to deaths in France is underestimated: 15% of French people declare it as the main cause of death in France, whereas it is one of the main causes after cancer, and the 1st cause of death worldwide.

Cardiometabolic diseases perceived as serious and on the rise
After explaining to respondents what cardiometabolic diseases are, 82% of them agreed that they are serious pathologies, 73% that they have increased over the last ten years, and 73% that they are widespread in the French population. On the other hand, the chronic nature of these pathologies seems to be largely unknown, with 57% of those surveyed explaining that they can now be cured.
It’s also worth noting that 1 in 2 French people believe that cardiometabolic diseases are primarily a matter of individual responsibility (50%), a result that goes hand in hand with the fact that they are perceived as being caused solely by lifestyle.
Well-identified preventive measures and risk factors
Another finding of the study is that cardiometabolic diseases are widely perceived by the French as being caused by lifestyle, with this cause being cited first (51%), far ahead of genetic predisposition (13%) or environmental factors such as pollution (6%).
As a corollary to this, the French believe that public authorities should above all fight against cardiometabolic diseases through campaigns to raise public awareness of lifestyle changes (36%). Conversely, only 15% mention scientific research and 12% the improvement of diagnostic tools, which are essential levers in the knowledge and fight against these diseases.
The French identify preventive health and diet measures fairly well: they believe that a balanced diet (94%), regular physical activity (93%), not smoking (92%) and not drinking too much alcohol (88%) are effective in preventing the onset of cardiometabolic diseases.
They also largely agree on the risk factors, citing being overweight (87%), being a smoker (86%), having a diet rich in fat, salt and sugar (85%) or a stressful lifestyle (82%).

More French people fear cardiovascular disease
The results of the study also show that judgments about the various cardiometabolic pathologies are ambivalent, probably because representations diverge according to the type of disease.
Cardiovascular diseases are now better identified as diseases of the cardiometabolic system, and give rise to a great deal of concern. This type of disease is by far the most worrying (50%), far ahead of hypertension (14%), diabetes (16%), obesity (11%), non-alcoholic fatty liver disease (6%) or hypercholesterolemia (3%).
As a corollary to this, the French feel that these are the diseases that should receive priority attention from the public authorities (46%), ahead of obesity (26%), diabetes (15%), rare metabolic diseases (7%), hypercholesterolemia (3%) and non-alcoholic fatty liver disease (3%).
A lack of information about cardiometabolic diseases
Just over a third of French people say they have already been informed about cardiometabolic diseases (37%). In detail, slightly more people over 65 (42%), wealthier people (48%) and donors to medical research (50%) say they have been informed.
Doctors are the main source of information about these diseases (57%), ahead of family and friends (37%), the media (29%) and government communication campaigns.
Who we are?
Created in 2011, IHU ICAN is a scientific cooperation foundation whose main objective is to develop tomorrow’s medicine to combat cardiometabolic diseases. Located in the heart of Europe’s largest public hospital, At the Pitié-Salpêtrière Hospital, the ICAN HCI draws on the expertise of its scientific community. (168 doctors, 261 researchers) and its 3 founders to carry out its missions: AP-HP (Assistance publique – Hôpitaux de Paris), Inserm (Institut national de la santé et de la recherche médicale) and Sorbonne University.
Any questions? Contact us:
- Francine TROCME
- Director of Communications and Patronage – IHU ICAN
- 06 81 64 97 88
- f.trocme@ihuican.org

Type 2 diabetes: link between aortic flow and epicardial fat discovered
Type 2 diabetes: link between aortic flow and epicardial fat discovered
A chronic disease on the rise, type 2 diabetes (T2DM) affects 3.5 million patients in France.
We now know that this pathology is a cardiovascular risk factor, leading in particular to an increase in aortic stiffness (the aorta, the body’s main artery, no longer has sufficient capacity to absorb the pulsation linked to the blood flow carried by the heart).
To improve early diagnosis of T2DM, ICAN IHU teams conducted a research project to analyze the link between aortic flow and epicardial fat in a population of type 2 diabetics from the MetaCardis* clinical trial.
Découvrez ci-dessous les conclusions de la publication scientifique ” Associations of aortic stiffness and intra-aortic flow parameters with epicardial adipose tissue in patients with type-2 diabetes “ parue le 26 mai 2023 dans Frontiers in Clinical Diabetes and Healthcare.
A study of aortic flow in patients with T2DM
With around 1 million people undiagnosed in France, T2D risk factors are relevant biomarkers for detecting metabolic severity and predicting unfavorable disease progression.

The aim of the project was to evaluate aortic flow parameters in patients with T2DM compared with healthy individuals, in order to assess the link with epicardial adipose tissue (EAT) accumulation as an index of cardiometabolic severity in diabetic patients.
- Inclusion of 36 T2DM patients and 29 healthy volunteers
- Cardiac and aortic MRI at the Pitié-Salpêtrière Heart Institute
What are the results?
In our study, aortic stiffness, represented by increased backflow volume and decreased distensibility, appears to be related to epicardial adipose tissue (EAT)volume in patients with type 2 diabetes (T2DM).
Epicardial adipose tissue (EAT), a biomarker of metabolic severity, was higher in T2DM patients than in controls, and correlated negatively with ascending aortic distensibility and positively with normalized return flow volume.
The study found that the left ventricular phenotype was characterized by concentric remodeling with a decrease in stroke volume index despite overall left ventricular mass within a normal range.
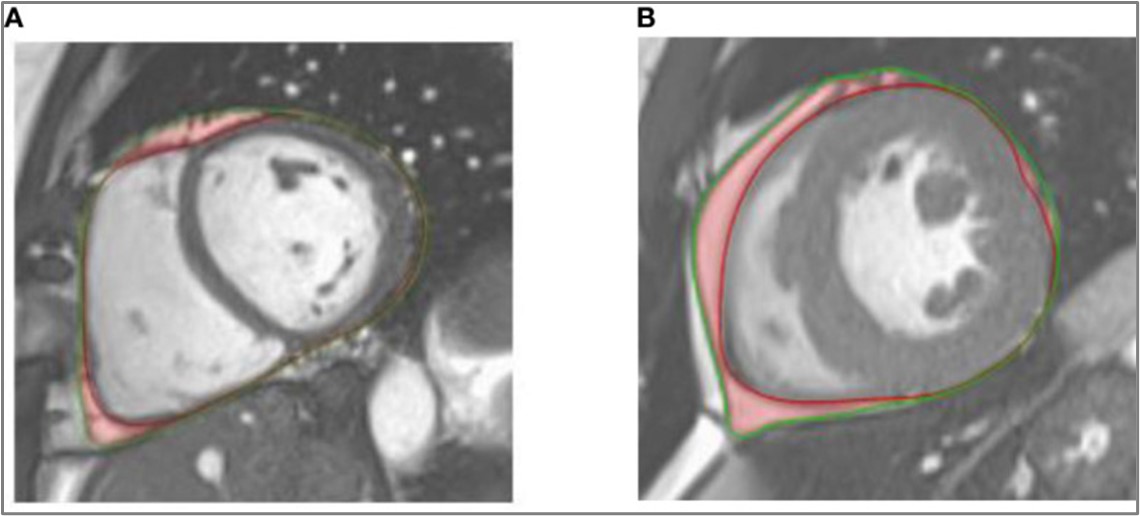
Caption: Illustration of epicardial adipose tissue (in red) on a cine-SSFP end-diastolic medioventricular short-axis image of a healthy control. (A) and a patient with type 2 diabetes (B). The epicardium and pericardium are highlighted by red and green lines respectively.
This observation should be confirmed in the future on a larger population taking into account other specific biomarkers of inflammation and using a longitudinal prospective study design.
The research project team
- Khaoula Bouazizi (ICAN Imaging – IHU ICAN, Laboratoire d’Imagerie Biomédicale – LIB, INSERM)
- Mohamed Zarai, Mikaël Prigent (IHU ICAN)
- Dr Abdallah Noufaily (Unité d’Imagerie Cardiovasculaire et Thoracique – ICT)
- Thomas Dietenbeck, Emilie Bollache, Nadjia Kachenoura (Laboratoire d’Imagerie Biomédicale – LIB, INSERM)
- Dr Toan Nguyen, Dr Valéria Della Valle, Pr Eléonore Blondiaux (AP-HP, Service de radiologie, Hôpital Armand-Trousseau)
- Pr Karine Clément, Pr Judith Aron-Wisnewsky (Nutriomics – Nutrition et obésité : approches systémiques, Centre de Recherche en Nutrition Humaine – CRNH)
- Pr Fabrizio Andreelli (IHU ICAN, AP-HP, Service de diabétologie, Hôpital Pitié-Salpêtrière)
- Pr Alban Redheuil (ICAN Imaging – IHU ICAN, AP-HP, Laboratoire d’Imagerie Biomédicale – LIB – INSERM, Unité d’Imagerie Cardiovasculaire et Thoracique – ICT)
*Funded by the European Union and coordinated by Inserm, MetaCardis (Metagenomics in Cardiometabolic Diseases) is a major innovative research project on the impact of changes in gut flora on the onset and progression of cardiometabolic and nutrition-related diseases. In 2012, it was deployed in 6 European countries with the support of a consortium of 14 partners. Know more.
How to optimize decision making for defibrillator implantation in hypertrophic cardiomyopathy?
How to optimize decision making for defibrillator implantation in hypertrophic cardiomyopathy?
Hypertrophic cardiomyopathy (HCM) is a genetic heart disease affecting around 120,000 people in France. It is a major cause of sudden cardiac death, particularly in athletes under 35.
Today, the only effective prevention of sudden cardiac death, apart from exercise restriction, is the implantable automatic defibrillator (ICD), but the indications for its implantation remain controversial.
To address this issue, the Pr Philippe Charron launches the project OPTIM-HCM. Its main objective is to improve decision-making in the field of ICDs by developing a new prediction model of sudden cardiac death in hypertrophic cardiomyopathy based on imaging and genetic biomarkers.
The context: how to improve the decision to implant an automatic defibrillator?
What is hypertrophic cardiomyopathy (HCM)? This disease is characterized by abnormal thickening of the heart muscle (myocardium), which stiffens the left ventricular muscle and thus impedes the heart’s pumping function.
- Symptoms: loss of consciousness, chest pain, shortness of breath, heart failure, etc.
- Possible complications: atrial arrhythmia (with risk of stroke) or ventricular arrhythmia (with risk of sudden death).
In view of the possible dramatic evolution of HCM, the identification of patients who should receive an implantable automatic defibrillator (ICD) is of paramount importance.
However, the indications for ICD implantation remain controversial. Coming from the American Society of Cardiology, the French National Authority for Health (HAS) and the European Society of Cardiology, the various recommendations are based on retrospective studies with significant limitations:
- Lack of predictive power of the features on which it is based,
- Failure to take into account recently identified MRI and genetic risk markers,
- Failure to take into account recently identified MRI and genetic risk markers,
- Failure to take into account the adverse effects of defibrillators,
- No medico-economic evaluation.
As a result, improvements in HCM management are urgently needed.
The OPTIM-HCM project: a prospective study to propose new recommendations
The main aim of the OPTIM-HCM research project is to improve decision-making in the field of automatic implantable defibrillators, by developing a new model for predicting sudden cardiac death in hypertrophic cardiomyopathy. This will reduce mortality and improve patients’ quality of life.
- The 1st prospective study to take into account recent risk markers such as magnetic resonance imaging (MRI) and genetics in a multivariate model, and to integrate potential adverse effects of ICDs into the overall decision-making strategy.
- Designed to propose a revision of international recommendations for defibrillator implantation in patients with HCM.
Progress of the OPTIM-HCM research project
Our hypothesis is that the data available and measurable non-invasively in vivo in MRI, are very under-exploited in this pathology, despite their richness and accuracy.
The OPTIM-HCM study therefore aims to generate detailed, innovative and multimodal complementary data using the latest imaging and genetic techniques, in order to evolve the sudden death risk score currently used for the decision to implant an automatic implantable defibrillator.
The project draws on the joint expertise of our ICAN Imaging Core Lab, part of the Pitié Salpêtrière cardiovascular imaging team (ICT) and the cardiovascular imaging team at the Laboratoire d’Imagerie Biomédicale (LIB, Sorbonne University, INSERM, CNRS), under the supervision of Pr Alban Redheuil, de Nadjia Kachenoura (DR INSERM) et Khaoula Bouazizi (IR INSERM).
By mid-2023, 600 cardiac MRI scans from some 30 centers in France have already been analyzed to study biomarkers of cardiac function, structure and morphology.
- Studied data: clinical data, MRI, echocardiography, genetic data, biological data
- Total study duration: 72 months
- Total budget: €1,063,698
- Project sponsor: Pr Philippe Charron, PU-PH, Head of the Genomics and Pathophysiology of Myocardial Diseases research team (UMR 1166/IHU ICAN) and Coordinator of the Reference Center for Hereditary and Rare Heart Diseases (Hôpital de la Pitié-Salpêtrière, AP-HP).
The essential role of our patrons
Le Triomphe du cœur, a private endowment fund for research into sudden death in adults, has joined forces with the ICAN IHU teams to support the OPTIM-HCM project.
We warmly thank this new patron for his commitment and confidence!
This project still needs support!
Contact Francine Trocmé (Director of Patronage and Communication IHU ICAN) to find out more about our research into hypertrophic cardiomyopathy, and how you can support us.
The SINBAD score, a useful tool for predicting major adverse foot events in diabetic patients?
The SINBAD score, a useful tool for predicting major adverse foot events in diabetic patients?
With diabetes on the rise worldwide, foot ulcers in diabetic patients are a major public health problem. In the United States, the cost is estimated at $80 billion every year.
To combat the progression of diabetic foot wounds, medicine recommends a a multidisciplinary approach, which generally makes it possible to reduce the risk of amputation by 45-55%: foot offloading, treatment of infection, septic surgery, revascularization, topical care, optimization of glycemic control, prevention of undernutrition…
The SINBAD score is an easy-to-use scoring system for monitoring patients with diabetic foot ulcers. However, its link with the occurrence of major adverse foot events (MAFEs) had not yet been studied.
The IHU ICAN teams therefore conducted a research project to assess the relationship between the SINBAD score and major adverse foot events in diabetic patients with foot ulcers. Publiées le 31 juillet dans Diabetes/Metabolism Research and Reviews, les conclusions sont résumées ci-dessous et consultables en version complète dans la publication “Use of the SINBAD score as a predicting tool for major adverse foot events in patients with diabetic foot ulcer : A French multicenter study”.
The aim of the research project
-
A scientific hypothesis to be confirmed
The primary objective of this study was to evaluate the relationship between the SINBAD score and the risk of occurrence of at least one of 8 defined major adverse foot events:
- Hospitalization,
- Septic surgery,
- Revascularization,
- Minor amputation,
- Major amputation,
- Death,
- Infection,
- And ulcer recurrence.
The study hypothesis was that a high SINBAD score could be a predictor of major adverse foot events.
The secondary objective was to evaluate the relationship between the SINBAD score and the occurrence of each adverse event.
-
What is the SINBAD score?
The SINBAD score system is quick and easy to use, requiring no special equipment other than a clinical examination, and containing all the information needed for analysis by the patient’s medical team. It describes the following characteristics of the wound and its local and general environment:
- S(ite): Wound location
- I(schemia): Ischemia
- N(europathy): neuropathy
- B(infection bactérienne) : infection
- A(rea): surface area
- D(epth): wound depth

Research project methodology
- Retrospective ancillary study.
- Inclusion of 537 patients aged over 18 years attending a diabetic foot ulcer service for less than 12 months in 6 French hospitals.
- Inclusion dates: between February 1, 2019 and March 17, 2019, and between February 1, 2020 and March 17, 2020.
- Assessment of the SINBAD score at inclusion, then a follow-up period of 5 to 6 months with a new assessment of the frequency of major adverse foot events.
Study results
- A low SINBAD score (0 to 3) was observed in 61% of patients and a high score (4 to 6) in 39%, with a major adverse foot event observed in 24% and 28% of patients respectively.
- Multivariate analyses showed a significant relationship between SINBAD score and major adverse foot events.
- The SINBAD score (continuous or dichotomous) at inclusion was also significantly associated with 6 of the 8 major adverse foot events.
The study proved that the SINBAD score could be used by the clinician, in addition to the simple prognosis of healing and amputation, to assess the risk of major adverse foot events: each SINBAD unit and an > score were associated with an increased risk of develop one of the 8 major adverse foot events.
The study concludes that the SINBAD score is a useful tool for improving foot ulcer management and predicting major adverse foot events (MAFEs).
It can be used in the same way as for the observation of major adverse cardiovascular events (MACE) in patients at cardiovascular risk.
Study participants
- Dr Georges Ha Van, Antoine Perrier, Service de diabétologie, AP-HP, Hôpital de la Pitié-Salpêtrière, Paris
- Dr Sophie Schuldiner, Service de Maladies Métaboliques et Endocriniennes, Centre Hospitalier Universitaire de Nimes
- Pr Ariane Sultan, Service endocrinologie, nutrition et diabétologie, CHU de Montpellier, PhyMedExp, INSERM U1046, CNRS UMR 9214, Université de Montpellier
- Dr Benjamin Bouillet, Service Endocrinologie et diabétologie, CHU Dijon Bourgogne / INSERM / Université de Bourgogne Franche-Comté, LNC UMR1231, Dijon
- Dr Jacques Martini, Service d’endocrinologie, CHU de Toulouse
- Dr Julien Vouillarmet, Service d’endocrinologie, CHU de Lyon
- Mehdi Menai, Aurélie Foucher, IHU ICAN, Fondation pour l’Innovation en Cardiométabolisme et Nutrition, Paris
- Pr Agnès Hartemann, Dr Olivier Bourron, Sorbonne Université, Service de diabétologie, IHU ICAN, AP-HP, Hôpital Pitié-Salpêtrière