Our research platform on chronic liver diseases
Chronic liver disease is now a major public health issue and a major cause of death worldwide.
The ICAN BioCell Human Liver Biology Platform aims to produce primary human liver cells (hepatocytes and all non-parenchymal cells) and to develop primary culture liver models in 2D or 3D (thin slices or spheroids) to study chronic liver diseases, in particular fibrosis and NASH.
Our platform is accessible to all academic research units and open to industrial projects.
Are you a patient?
Thanks to a partnership between AP-HP, INSERM, Sorbonne University and the ICAN IHU, the ICAN BioCell Human Liver Biology platform combines technological innovation with the maintenance of a collection of samples and products from the human body.
Thank you to all the patients who have agreed to take part in the ICAN BioCell Human Liver Biology collection! Research into chronic liver disease is making progress thanks to you. If you have made a donation to the Human HepCell collection and wish to object to any or all of the research, you can contact the surgeon who operated on you, or inform protection.donnees.dsi@aphp.fr.

Our catalogue
BioCell Human Liver Biology offers a catalogue for the production and sale of different liver cell types:
- Fresh human hepatocytes (HH)
- Hepatic myofibroblasts humanis (HLMF) from normal liver, NASH, fibrosis and cirrhosis
- Non-parenchymal cells (NPC)
- Endothelial cells, kupffer cells, immune cells, etc
- Bile cells from the gall bladder
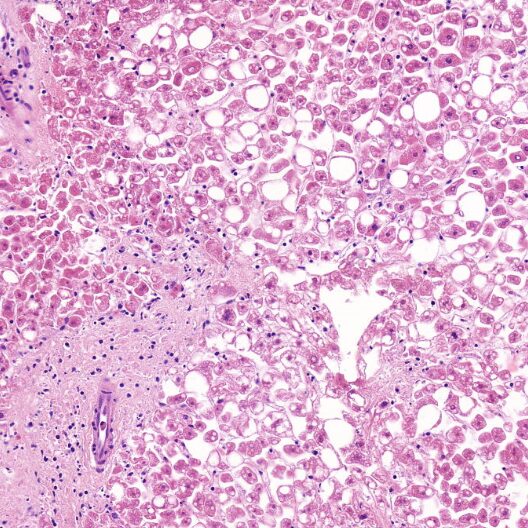
- Precision cut liver slices (PCLS), healthy or pathological (NASH, fibrosis, cirrhosis). See the scientific publication.
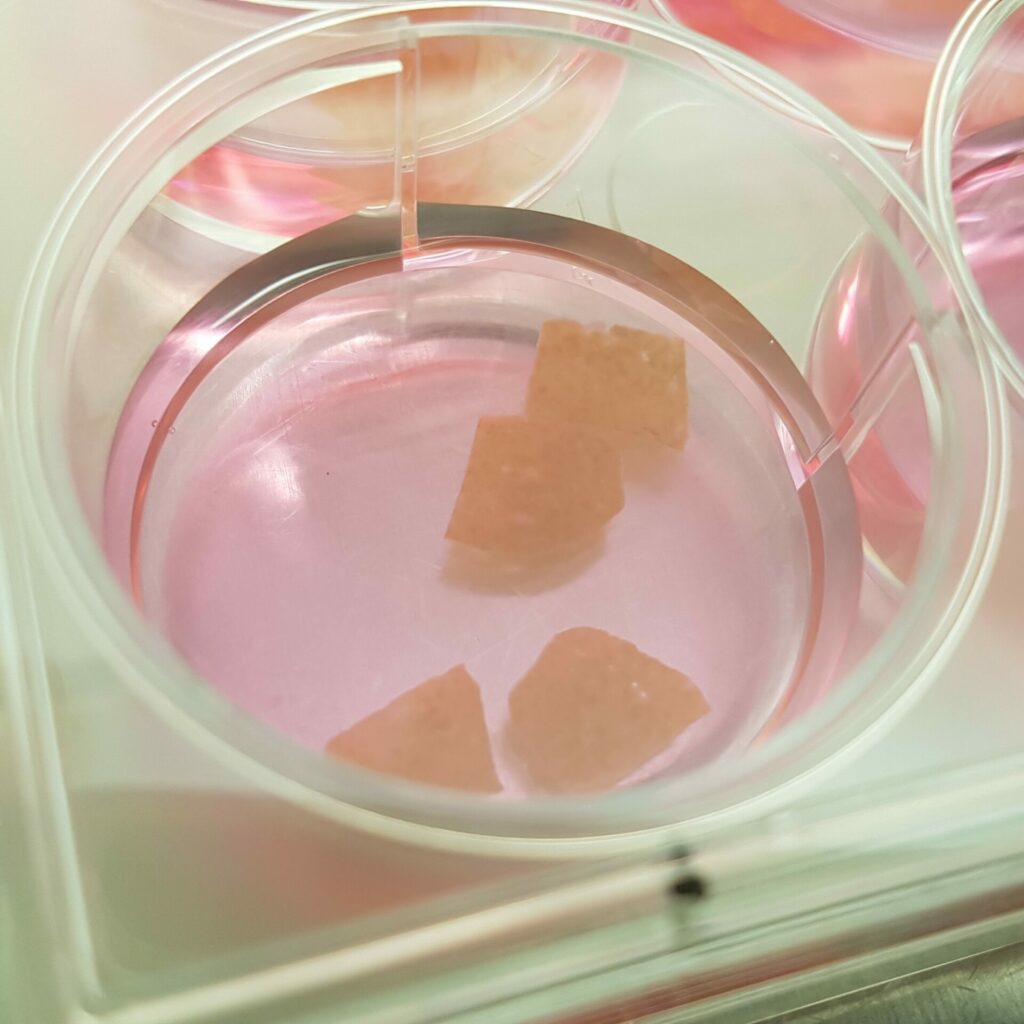
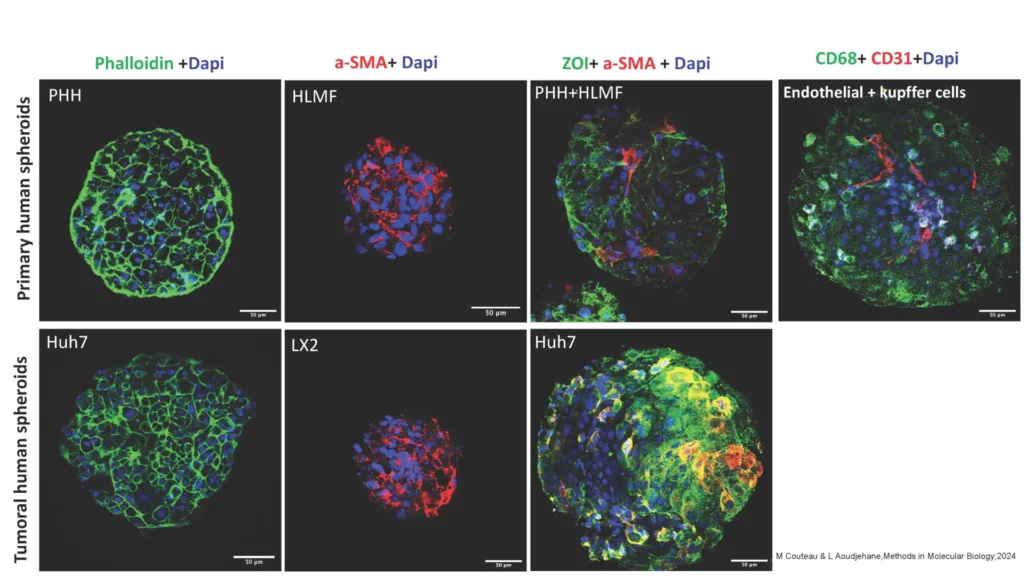
- Hepatic spheroid/organoid model. See scientific publication.
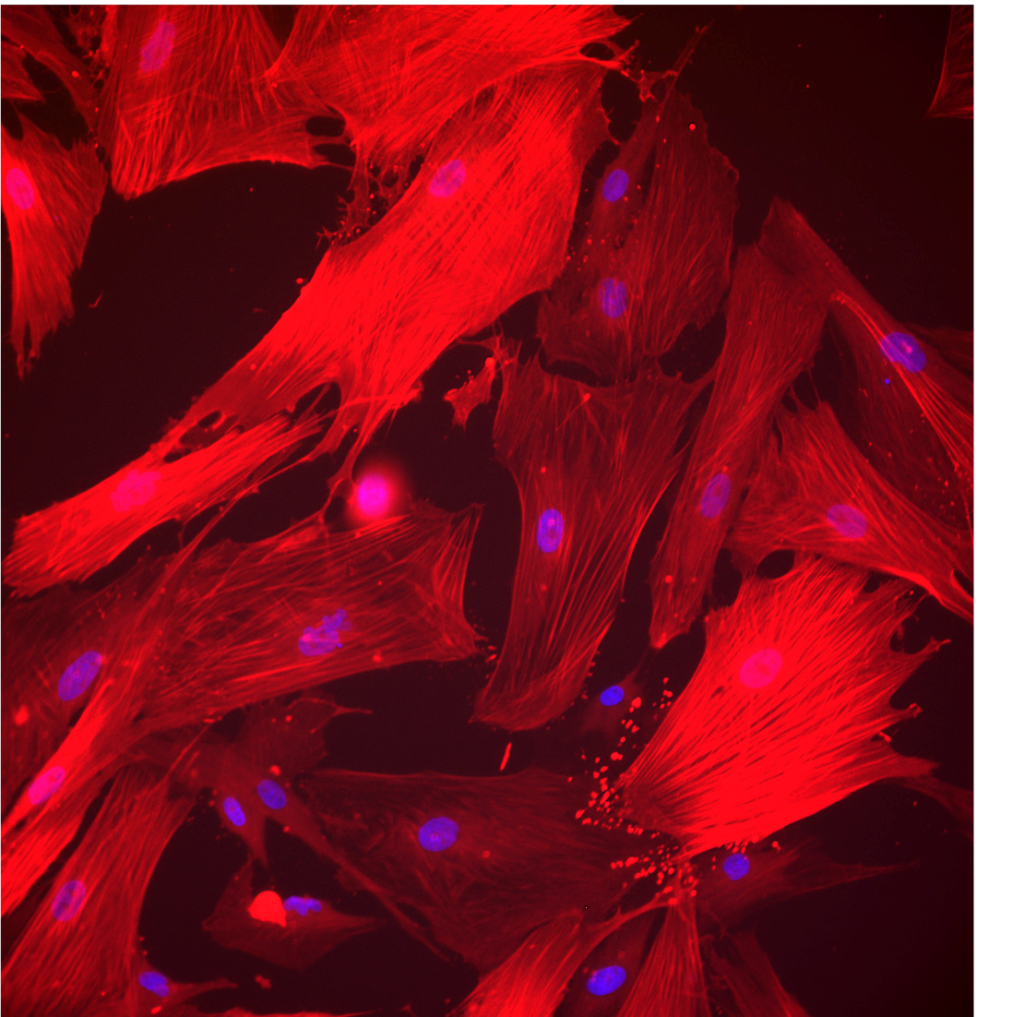
Our services
ICAN BioCell – Human Liver Biology also offers a range of research services:
Fibrosis
We have evaluated the sensitivity of hepatic myofibroblasts from different patients (normal and cirrhotic) to antifibrotic molecules using our Quanti-Fibrosis® platform. Our data established the differential efficacy of these molecules, revealing the impact of human polymorphism and disease background on drug sensitivity
Induction of steatosis in vitro in normal HH or PCLS with fatty acids.
NASH
we have 2D and 3D models to study hepatic steatosis associated or not with inflammation
- Hepatocytes (HH) or PCLS from steatotic livers
- Induction of steatosis in vitro in normal HH or PCLS with fatty acids
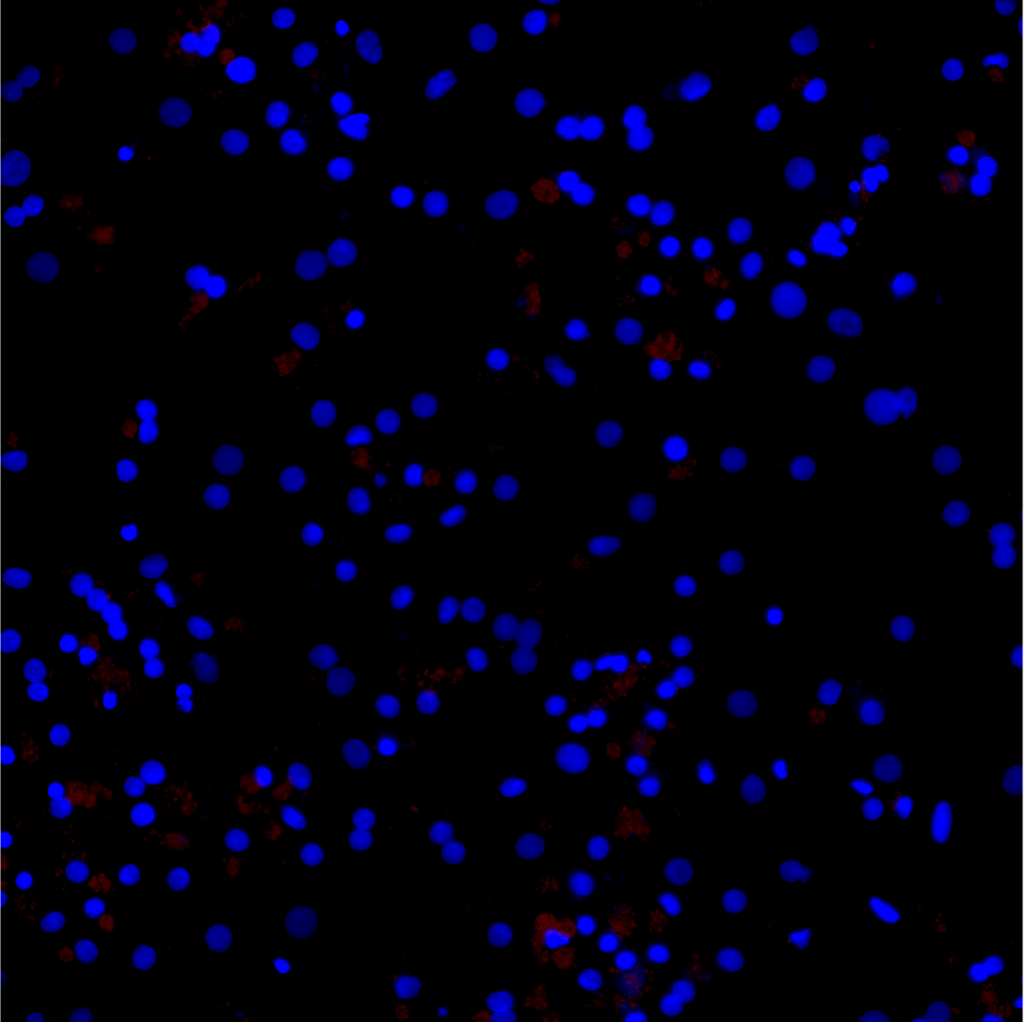
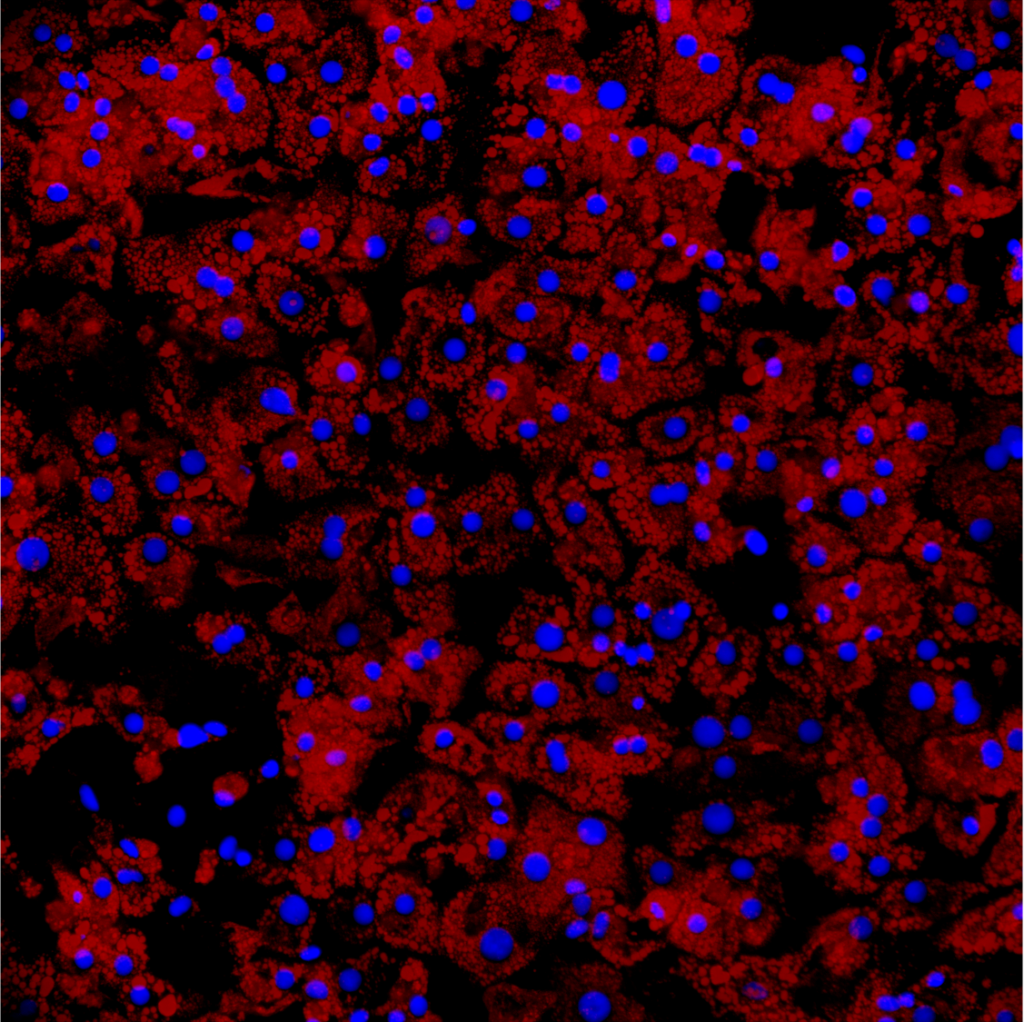
- Lipid metabolism (triglyceride assay, RedOil staining, genes involved in lipid metabolism (beta oxidation, lipid export, autophagy, lipogenesis, etc.)
- Functional hepatic metabolism (albumin, CYP3A4 activity, ABC transporters, etc.)
- Viability and toxicity (MTT, ATP and HDL) and oxidative stress (ROS detection), pro-inflammatory cytokines (QPCR and ELISA)
Contact
ICAN BioCell Human Liver Biology platform
Sorbonne University Faculty of Medicine
Pitié6Salpêtrière Hospital
91 boulevard de l’Hôpital
75013 Paris, France
Scientific Advisory Board
- Pr Filomena Conti – Senior Scientific Advisor
- Dr Fabienne Foufelle
- Dr Laura Fouassier
- Prof. Mathieu Hautefeuille
- Dr Wilfried Le Goff
- Prof. Vlad Ratziu
- Prof. Olivier Scatton
Major publications ICAN BioCell Human Liver Biology
Focus on 2 major publications
29/04/2020
Liver transplantation (LT) is now the recognised treatment for end-stage chronic liver disease. There is currently a shortage of organs and ‘marginal’ grafts, particularly steatotic (fatty liver) grafts, are increasingly being used to try to limit this shortage. Steatosis is the main reason why liver transplants are rejected by TH teams, as it is responsible for liver dysfunction after transplantation. The use of steatotic grafts could go some way to reducing the shortage of grafts currently affecting patients waiting for TH. Our strategy is to treat these ‘candidate’ grafts prior to implantation using normo-thermal perfusion (physiological temperature 37°C), which would improve tolerance to ischaemia and extract lipid vacuoles from the hepatocytes. Our ‘degreasing’ method involves acting directly on the excessive accumulation of lipids. In this study, we devised a ‘degreasing’ cocktail to remove the lipids stored in steatotic hepatocytes. To do this, we created a cocktail composed of molecules designed to activate each stage of intra-hepatocyte lipid metabolism until the lipids are removed from the cell.
21/11/2019
Hepatic steatosis is the accumulation of fat in the liver. Steatosis is very common(25% of the French population is affected) and is very often associated with overweight and type 2 diabetes. Often asymptomatic and of no consequence, the accumulation of fat is responsible in 30% of cases for inflammatory lesions of the liver (non-alcoholic steatohepatitis or NASH), which can lead to non-alcoholic cirrhosis and liver cancer. The inflammation and death of liver cells in NASH is initially compensated for by the liver’s regenerative capacity. However, in the context of chronic liver damage, as observed in NASH, these healing capacities are deleterious and contribute to the destruction of the liver (fibrosis). One of our current research objectives is to identify the mechanisms behind inflammation and cell death in NASH in order to propose effective treatments. Our team has recently demonstrated, through work in mice and on human liver cells , that blocking cell death in NASH reduces inflammation and abnormal scarring of the liver and limits the accumulation of fat in the liver.
All publications
06/06/2025
06/02/2024
07/2023
21/06/2023
An Efficient 5-Aminolevulinic Acid Photodynamic Therapy Treatment for Human Hepatocellular Carcinoma
10/02/2022
18/03/2021
Ex-Vivo Pharmacological Defatting of the Liver: A Review
02/03/2020
Cancer-associated fibroblasts in cholangiocarcinoma
12/10/2020
19/05/2020
28/10/2019
22/10/2019
11/08/2016
Heregulin-1ß and HER3 in hepatocellular carcinoma: status and regulation by insulin
07/03/2016
Development of an in vitro model to test antifibrotic drugs on primary human liver myofibroblasts.
27/07/2015
25/10/2013
30/04/2013
09/11/2012
10/03/2012
Epidermal growth factor receptor and HER-3 restrict cell response to sorafenib in hepatocellular carcinoma cells







