New interfaces in the biology of cardiometabolic diseases
Cardiovascular diseases linked to diabetes, atherosclerosis, obesity and liver diseases such as NASH are on the increase in France and in all developed countries, and are now grouped together under the concept of cardiometabolic diseases. The scientific basis for this concept lies in the many interfaces between metabolism and the organs: microbiota, adipose tissue and the immune system. As a result, cardiovascular and metabolic diseases can no longer be considered as the disease of a single organ, but require a multi-organ, systemic approach that also takes into account the environment, nutrition and physical activity.
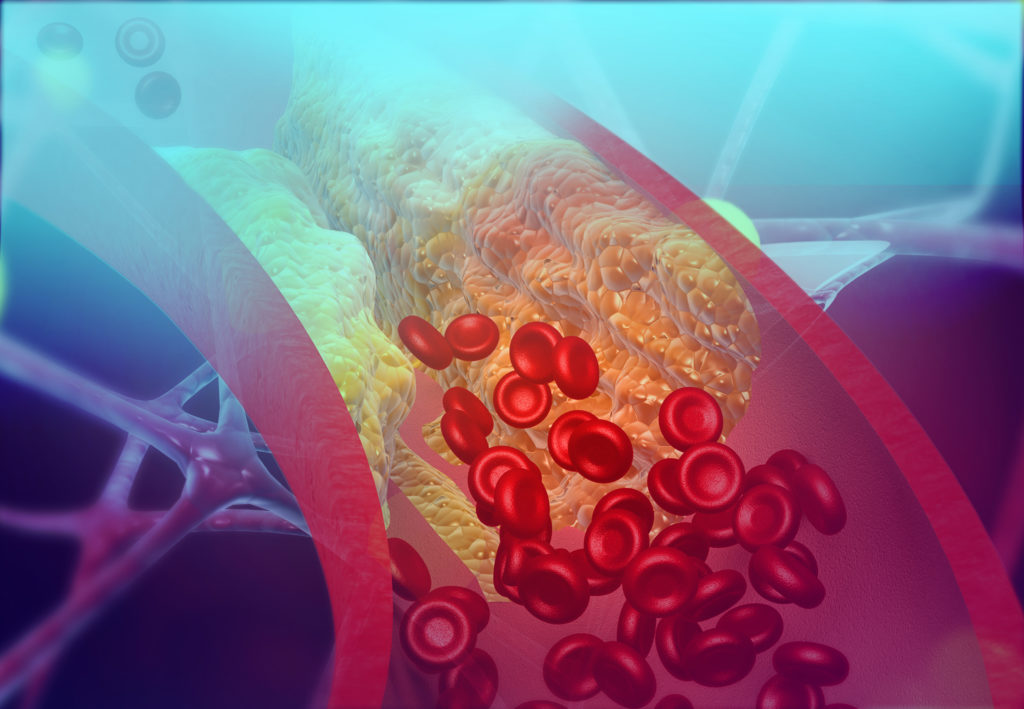
Cholesterol
Historically, cholesterol metabolism was the first interface described between metabolism and cardiovascular disease, particularly those linked to atherosclerosis, which is responsible for the formation of atherosclerotic plaque in the lumen of blood vessels.
For many years, teams at the IHU have been studying the transport of cholesterol from its digestive absorption and metabolism in the liver through to exchanges with the body’s cells. Their work has helped to establish the atheroprotective role of HDL particles , known as ‘good cholesterol’. Precise knowledge of cholesterol transport and metabolism has made it possible to identify new biomarkers, for example, for predicting outcomes after myocardial infarction, and new therapeutic targets.
Adipose tissue
Fatty tissue is one of the main interfaces between metabolism and organs in general, and the heart and blood vessels in particular. A distinction is made between cutaneous fatty tissue and visceral tissue around organs, particularly the heart and blood vessels. Visceral fatty tissue has a number of biological functions: firstly, it is a source of fatty acids necessary for metabolism, for example in the heart muscle. Visceral fatty tissue secretes numerous peptides that act locally or remotely, like hormones, on the inflammatory state or oxidative stress in tissues.
The IHU ICAN teams were pioneers in describing the role played by fatty tissue around the heart in the risk of the most common cardiac arrhythmia, atrial fibrillation. They showed that this fatty tissue could in certain circumstances promote fibrosis of the atrial myocardium, a mechanism of arrhythmias.
They identified the origin of this cardiac fatty tissue , which comes from the differentiation of progenitor cells resident in the outer leaflet of the heart, the epicardium. The recruitment of progenitor cells and their differentiation into fat cells occur during various cardiac stresses: work overload or metabolic stress. These discoveries have opened up new research perspectives on cardiac arrhythmias and in particular on the impact of metabolic diseases, obesity, metabolic syndrome and diabetes, on the accumulation of cardiac fatty tissue and its biological activity (Chua W et al. BMC Cardiovasc Disord. 2019).
The microbiota
The IHU’s specialist nutrition teams have helped to identify the relationship between cardiovascular disease and the intestinal microbiota , and in particular how intestinal dysbiosis promotes metabolic disease. Microbial genetic richness is lowered during obesity and metabolic diseases, while high microbial richness is associated with healthy eating habits (Dao Front Physiol 2019).
The Metacardis cohort, set up as part of a European FP7 project and co-funded by the ICAN IHU, has shown that statin treatment improves the decline in microbial gene diversity in patients with cardiometabolic diseases (Vieira-Silva S et al Nature 2020). Imidazole propionate derived from bacterial metabolism of certain nutrients is increased in type 2 diabetic patients and associated with the presence of microbial dysbiosis (Molinaro et al Nature communication 2020).
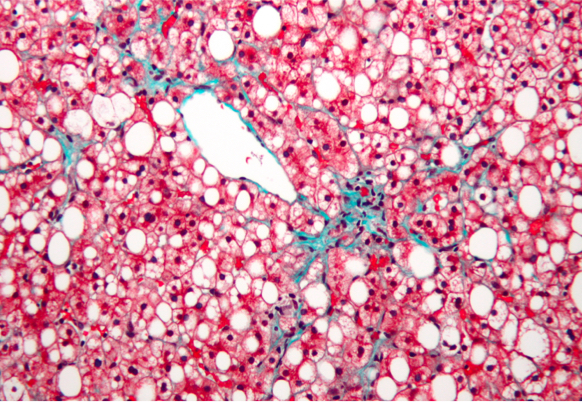
The liver
The liver is at the centre of the metabolic syndrome and is involved in the metabolism of carbohydrates and lipids. NASH (metabolic steatopathy) is one of the hepatic manifestations of insulin resistance, with consequences not only for the liver (progression of fibrosis, cirrhosis and its complications) but also for cardiometabolic risk factors (cardiovascular disease, diabetes, etc).
As a result, the liver is at the heart of ICAN’s areas of interest in a cross-disciplinary approach that links the various pathologies associated with metabolic syndrome and cardiovascular disease. ICAN is the promoter of an innovative, complex and multidisciplinary care circuit dedicated to patients with NASH (the NASH Clinic), enabling precision phenotyping and a personalised therapeutic approach tailored to each patient.
ICAN actively supports the development of new molecules to treat NASH. ICAN is involved in several European consortia focusing on NASH – LITMUS and EU-PEARL (funded by IMI2 and EFPIA). The hepatology team within the ICAN IHU has carried out important work focusing on the natural history of NASH (Pais J Hepatol 2013), the relationship with cardiovascular disease (Pais J Hepatol 2016, Pais Hepatology 2019 and the CORO-NASH project) and more recently, in collaboration with the AP-HP, the relationship between the liver and COVID-19 (STRAT-COVID project). Finally, the IHU is involved in fundamental research aimed at exploring the pathophysiological mechanisms involved in NASH and the progression of fibrosis (ceramides, sphingolipids, apoptosis pathways and cell death) (work by C Housset, F Foufelle, J Gautheron, HumanHepCell).