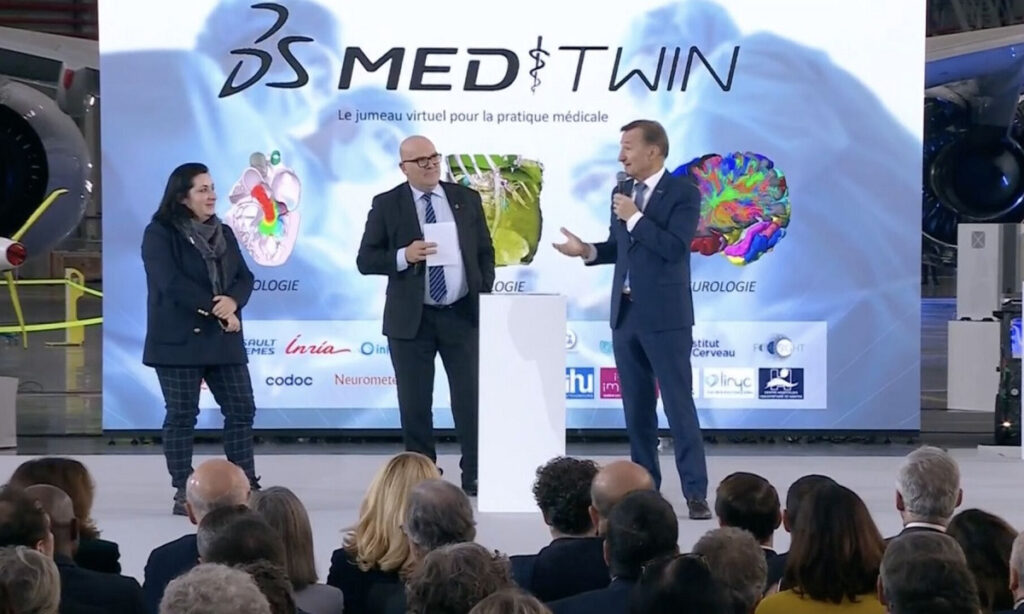
How can the use of virtual twins improve the quality of care, making healthcare safer and more accessible to all? This is the mission of MEDITWIN, a consortium made up of 7 University Hospital Institutes (IHUs) including the ICAN IHU, Nantes University Hospital, Inria, associated start-ups and Dassault Systèmes, announced on Monday 11 December 2023 in the presence of French President Emmanuel Macron.
Using personalised virtual twins of organs, metabolism and cancerous tumours, this innovative project will make it possible to diagnose the risk of cardiovascular disease for high-risk patients, and to help specialist doctors choose treatments for effective patient follow-up.
The MEDITWIN initiative will be developed over 5 years, from 2024 to 2029. The partners’ investment in this project will be financially supported by the French government as part of France 2030.

What medical need does MEDITWIN meet?

“Digital twins open up the prospect of a new form of precision and preventive medicine for cardiometabolic diseases by determining the future risk of developing cardiovascular disease for each individual on the basis of their risk factors, in particular their genomic signature. Such a project requires a change of scale in research, which has now been achieved thanks to the project led by Dassault Systèmes, with all the MEDITWIN partners on board”
Prof. Stéphane Hatem, Director General of the ICAN IHU and Director of UMR 1166 Cardiovascular and Metabolic Diseases
Cardiometabolic diseases, also known as cardiovascular-metabolic diseases, refer to a group of interconnected disorders that affect the heart and metabolic system. They mainly include: cardiovascular diseases that affect the heart and blood vessels (coronary heart disease, hypertension, etc.) and metabolic disorders (type 2 diabetes, obesity, hypercholesterolaemia, steatotic liver disease – MASH-(NASH)).
Cardiovascular disease is the world’s leading cause of death, accounting for almost 18 million deaths every year. In France, it is the 2nd leading cause of death in the general population, with almost 150,000 deaths each year, and the 1st cause in women. More than 15 million people are treated in France for cardiovascular disease or diabetes, at an annual cost of more than €17 billion.

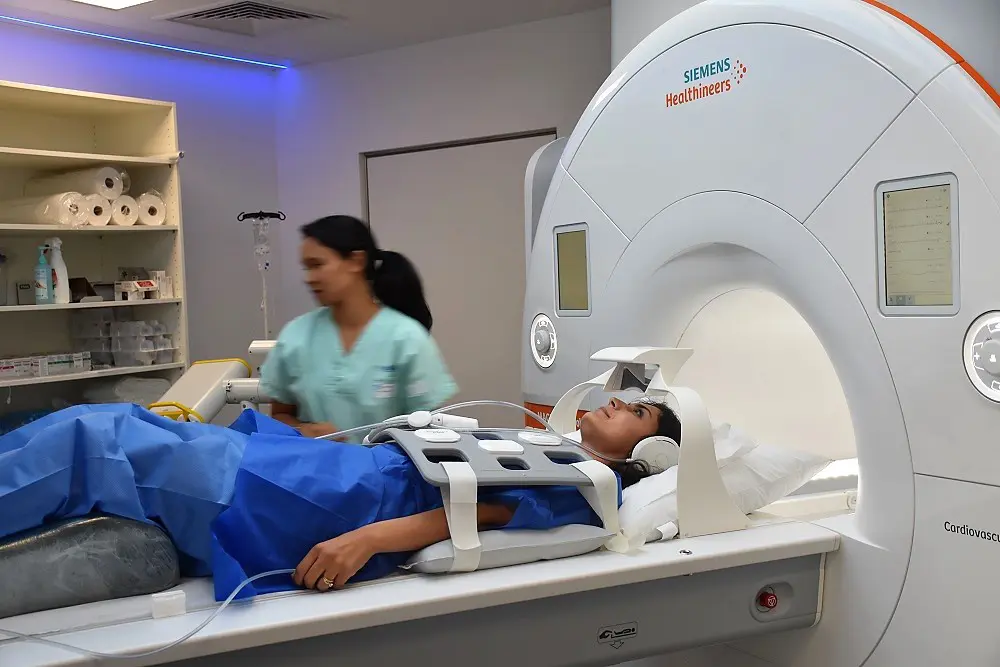
To date, the level of cardiovascular risk is assessed in all symptomatic and asymptomatic patients at risk identified by their doctor.
The association between certain classic biological markers (classic lipid profile, LDL-cholesterol) and the risk of cardiovascular disease is indisputable. However, to date, the use of these markers in medical practice does not allow this population to be finely stratified.
Without predictive tools, it is difficult to individualise patient follow-up and choose the most appropriate treatments, as well as the optimum doses, while reducing the side-effects associated with treatment (statins).
Technical advances not yet systematised
Scientific and biomedical research has made significant advances in this area, with plans to integrate other markers based on imaging and Omics data (metagenomics, metabolomics, lipidomics, proteomics).
Advances in imaging techniques, such as thoracic scans (large and small arteries), now enable non-invasive, detailed analysis of vascular damage in patients with no known cardiovascular disease.
By revealing the progression, stabilisation or regression of the disease and predicting the quality of response to treatment, these new indicators are becoming a practical means of applying prevention and targeted therapies according to the patient’s profile and biology.
Familial hypercholesterolaemia (FH) is a major cause of premature cardiovascular disease and represents a model of accelerated pathological progression. Following the trajectory of these patients is therefore an opportunity to gain access to risk prediction indicators over a limited period.
Recent data have shown that, even in the case of familial hypercholesterolaemia, the use of non-invasive coronary imaging can predict the incidence of cardiovascular events in asymptomatic subjects, with a high predictive power over a follow-up of less than 3 years.
However, these techniques are not fully integrated into decision-making algorithms and their use in clinical practice is not systematic.
The use of virtual twins, for a better treatment strategy
With this use case for virtual twins, the ambition of the MEDITWIN consortium is to develop a personalised predisposition diagnostic service for cardiovascular diseases and to select the best treatment strategies, while integrating the patient’s environment such as his intestinal microbiota and nutrition.
The predictions of such twins in terms of trajectories and improvements in these trajectories could support the practitioner in the management of the patient, and also make the patient’s future more tangible in the absence of intervention in order to improve adherence to treatment. It will also be possible to measure the impact of the proposed solutions in terms of reducing the number of patients with cardiovascular events (coronary, cerebrovascular, arterial, etc.).
This will have a positive impact on patients, who will benefit from treatment tailored to their individual risk and directly linked to the progress of their disease, and on practitioners, who will be able to take advantage of tools for identifying and managing chronic diseases at an early stage.
MEDITWIN will therefore make it possible to establish a model of digital twins to monitor changes in the individual cardiovascular risk of patients with familial hypercholesterolaemia using a selection of relevant biomarkers.
Digital twins will make it possible to clarify the cause-and-effect relationship between micro- and macro-circulation in a healthy cardiovascular system, and then to gain a better understanding of the pathophysiological mechanisms involved in ageing, hypertension, diabetes, etc.







