Parental imprinting is an epigenetic mechanism that leads to monoallelic expression of a subset of genes depending on their parental origin. Parental imprinting diseases (Imprinting Disorders, ID), caused by disruptions of imprinted genes, are a group of rare congenital diseases that mainly affect growth, metabolism, and development.
To model the pathophysiology of parental imprinting diseases, a research team made up of physicians, researchers, and PhD students from Inserm, Sorbonne University, and IHU ICAN developed a new cellular approach. Published on December 28, 2022 in the journal Clinical Epigenetics, this study is entitled “Maintenance of methylation profiles in imprinting control regions in human induced pluripotent stem cells”.

What are the objectives of this approach?
To date, there is no reliable model to study the pathophysiology of parental imprinting diseases in humans, the first step toward developing appropriate therapeutic strategies. Human induced pluripotent stem cells (hiPSCs) are a promising cellular approach for modeling human diseases and complex genetic disorders.
However, aberrant hypermethylation of imprinting control regions (Imprinting control regions, ICR) can occur during the reprogramming process and subsequent hiPSC culture. Consequently, the research team tested different reprogramming and hiPSC culture conditions and carried out an in-depth methylation analysis at imprinting control regions, with the aim of developing a cellular model to understand parental imprinting diseases.
What are the study results?
Researchers assessed methylation at seven imprinted loci in hiPSCs before differentiation, at different passages of cell culture, and during chondrogenic differentiation. As already described in the literature, hypermethylation was identified at the imprinted regions 11p15 H19/IGF2:IG-DMR and 14q32 MEG3/DLK1:IG-DMR, regardless of the reprogramming method and the cells of origin.
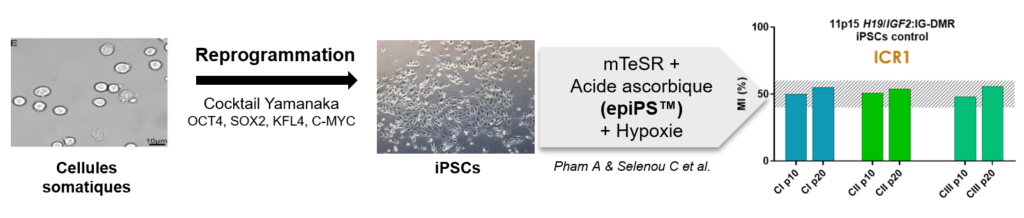
Hypermethylation at these two loci led to a loss of parental imprinting, with biallelic expression of the imprinted genes IGF2 and DLK1, respectively in the 11p15 and 14q32 regions. Development of the epiPS™ culture medium combined with culturing the cells under hypoxia made it possible to correct hypermethylation at H19/IGF2:IG-DMR and MEG3/DLK1:IG-DMR and restore parental imprinting, while preserving the proliferation and pluripotency qualities of these stem cells.
What are the conclusions?
An extensive quantitative analysis of imprinting control region methylation found hypermethylation at certain ICRs (those methylated on the paternal allele), in hiPSCs associated with a loss of imprinting in these regions.
The epiPS™ culture medium and hypoxic culture of hiPSCs made it possible to restore balanced methylation at these loci in controls. The research team also showed that methylation was disrupted in hiPSCs derived from a patient, thereby recapitulating the molecular abnormality responsible for their disease.
hiPSCs, cultured with this new protocol, therefore offer very promising prospects for differentiation into cells of interest involved in the phenotype of patients with parental imprinting disorders, to understand the pathophysiological mechanisms and consider therapeutic targets.
Stakeholders involved in the project
This project is supported by:
- IHU ICAN
- Sorbonne University
- Inserm
Stakeholders and authors of this study:
- Prof. Irène Netchine, MD-PhD, Head of the “IGF System and Fetal and Postnatal Growth” team at Sorbonne University/Inserm/AP-HP
- Dr Aurélie Pham, Clinical Assistant and PhD at Sorbonne University/Inserm/AP-HP
- Céline Selenou, PhD student at Inserm
- Dr Eloïse Giabicani, Associate Professor and Hospital Practitioner at Sorbonne University/Inserm/AP-HP
- Dr Vincent Fontaine, PhD at IHU ICAN
- Sibylle Marteau, iPS Cell Culture Assistant Engineer at IHU ICAN
- Dr Frédéric Brioude, Associate Professor and Hospital Practitioner at Sorbonne University/Inserm/AP-HP
- Dr Laurent David, Associate Professor and Hospital Practitioner at Nantes University
- Prof. Delphine Mitanchez, MD-PhD, Sorbonne University/Inserm
- Dr Marie-Laure Sobrier, Research Scientist at Inserm







