Homozygous familial hypercholesterolemia (HFHo) is an extremely rare genetic disorder that affects approximately 1 in 160,000 to 300,000 people. Its consequences include major cardiovascular complications such as angina pectoris, myocardial infarction, aortic stenosis, or sudden death in the early years of life. Without effective treatment, life expectancy rarely exceeds 30 years.
To respond to this cardiovascular emergency, the ICAN IHU is setting up the IDEAL-HFHo project, led by Dr. Antonio GALLO. It aims to evaluate the impact of an innovative treatment, Evinacumab, in real-world conditions.
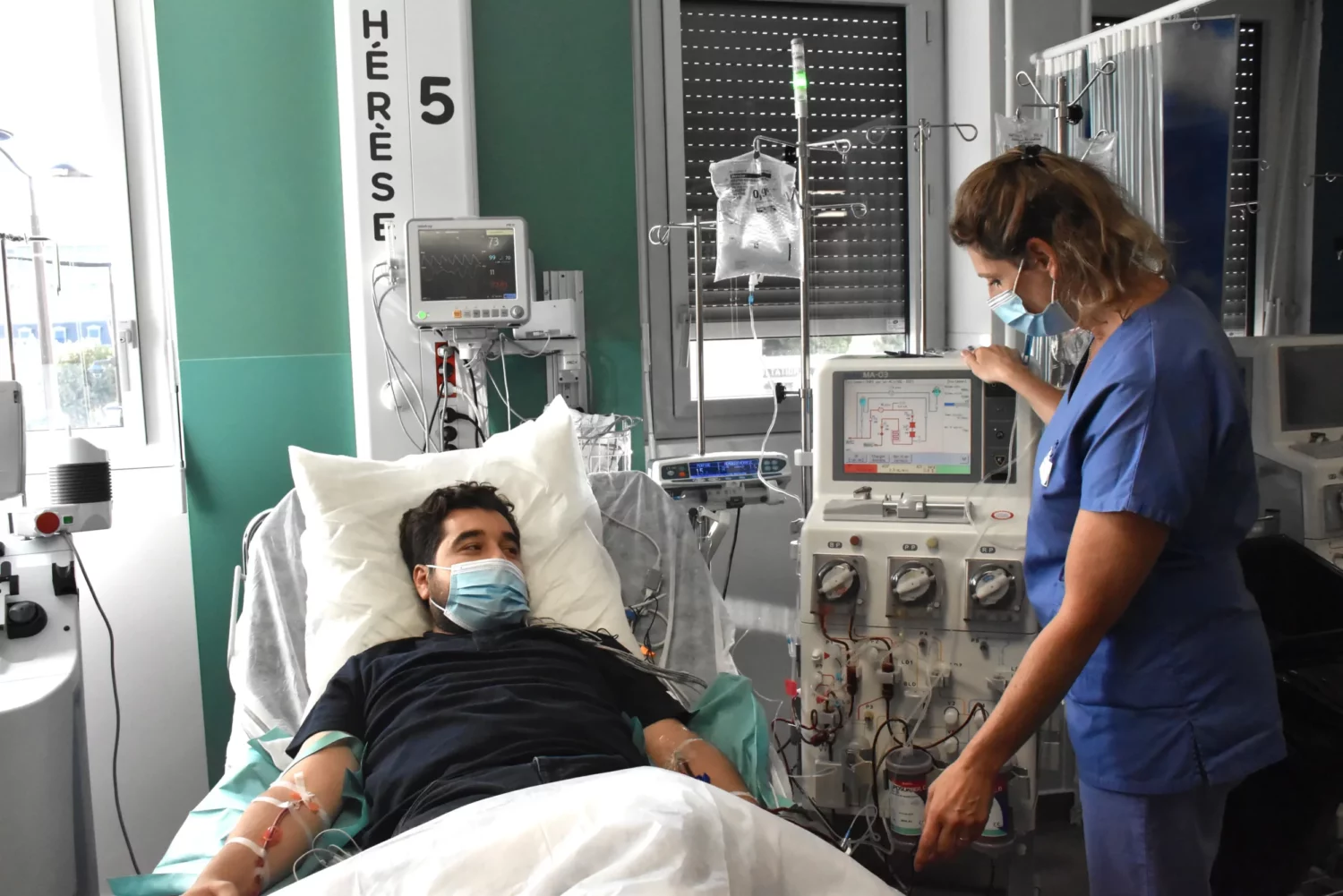
A vital issue for patients at high cardiovascular risk

Homozygous familial hypercholesterolemia (HFHo) causes extremely high levels of LDL cholesterol (“bad cholesterol”) from childhood onwards, often ten times higher than normal.
The result is rapid accumulation of plaque in the arteries (atherosclerosis), leading to major cardiovascular complications. These patients may suffer from angina pectoris, myocardial infarction, aortic stenosis, or sudden death in the first years of life. Several reported cases show rapid progression of the disease, with deaths occurring before the age of 5.
Treatments still very inadequate
Despite the existence of powerful lipid-lowering treatments (statins, ezetimibe, apheresis), the majority of patients are unable to achieve LDL cholesterol levels low enough to be protected. Apheresis—a complex technique, comparable to dialysis—is the standard treatment in the most severe cases. However, it is restrictive, not widely available, and poorly tolerated in the long term. In fact, less than 30% of patients worldwide have access to apheresis.
New therapies, such as PCSK9 inhibitors, have shown limited results in HFHo, as their effectiveness depends on the presence of a functional LDL receptor—which is precisely what these patients lack. Thus, treatment options remain very limited, especially for the most severe forms of the disease.
An urgent need for effective alternatives
The severity of the disease, its early impact on the cardiovascular system, and the relative failure of existing treatments underscore the urgency of finding new, effective, and well-tolerated strategies capable of reducing not only LDL cholesterol but also the actual cardiovascular risk in these young patients.
Any progress in this area can radically change the life trajectory of people who are currently condemned to premature death, offering them the chance to live longer, healthier lives with fewer heavy and invasive treatments.
The IDEAL-HFHo project: evaluation of an innovative treatment, Evinacumab
Given the cardiovascular urgency posed by homozygous familial hypercholesterolemia (HFHo), this research project aims to evaluate the impact of an innovative treatment in real-world conditions: Evinacumab, a recently developed monoclonal antibody targeting the ANGPTL3 protein. This protein targets the metabolism of triglycerides and HDL cholesterol (“good cholesterol”).
Treatment with Evinacumab is based on a mechanism that is independent of the conventional cholesterol elimination pathways, which is not yet fully understood, and is currently a promising option for the most severely affected patients, for whom current treatments are ineffective.
The main objectives of the project
Assess the impact of treatment on HDL cholesterol (the “good” cholesterol): quantity, quality, and protective functions (particularly its ability to remove excess cholesterol from the arteries and its anti-inflammatory properties).
Analyze the progression or regression of cardiovascular lesions (atheromatous plaque, perivascular inflammation, epicardial fat, calcifications) using high-precision imaging tests (coronary CT angiography).
Study the effects of treatment on biological markers of cardiovascular risk using OMICS analyses (lipidomics, transcriptomics, metabolomics).
Assess changes in patients’ quality of life, particularly for those who could hope to reduce or stop apheresis thanks to this treatment.
The project timeline
This national, multicenter, non-interventional clinical project (conducted in Paris, Marseille, and Lyon) is following two dozen adult patients with HFHo over a period of seven years (2025-2031). Its aim is to measure in detail the effects of treatment with Evinacumab on cardiovascular health, blood lipids, and quality of life.
The project is based on:
- Regular clinical follow-ups (1 month, 3 months, 18 months, 36 months, and 60 months), incorporating medical, biological, and imaging data;
- In-depth blood tests to explore the molecular mechanisms linked to the effects of treatment;
- Personalized monitoring of patients’ quality of life, with questionnaires and in-depth interviews;
- A specific analysis for patients currently undergoing apheresis, to measure the potential benefits in terms of therapeutic burden and chronic inflammation.
Expected results
The project addresses a key question: can treatment with Evinacumab significantly alter the cardiovascular progression of HFHo patients, beyond simply lowering LDL cholesterol? In other words, does it actually reduce the risk of heart attack, premature death, or dependence on heavy treatment?
The expected results could redefine the treatment of the most severe forms of this disease, with concrete consequences for survival, quality of life, and the reduction of inequalities in access to intensive care such as apheresis.
Become a patron of the IDEAL-HFHo project!
The IDEAL-HFHo project needs the support of sponsors and donors to accelerate its implementation and its medical and societal impact at the national and international levels.
Why support the IDEAL-HFHo project?
An integrated and innovative approach
homozygous familial hypercholesterolemia (HFHo), as part of an ambitious translational research program and a high level of expertise.
Support from a cohort of patients unique in France
and the use of cutting-edge technologies (OMICS, quantitative imaging, functional biology of HDL).
A multidimensional approach
unparalleled internationally in terms of both the wealth of data generated and the national coordination between expert centers, making IDEAL-HFHo a project that is unique, competitive, and has significant potential for clinical impact.
Close collaboration between multiple disciplines
with the collaboration of fundamental and translational research teams specializing in lipid metabolism, transcriptomics, inflammation, and vascular biology (Inserm UMRS 1166, ICAN, Inserm UMR 1146) and clinicians specializing in lipidology, internal medicine, endocrinology, cardiology, and nutrition from three major hospitals in France:
- Paris – Pitié-Salpêtrière Hospital (Lipidology and Cardiovascular Prevention Department)
- Marseille – La Conception Hospital (Nutrition Department)
- Lyon – Lyon Civil Hospitals (Nutrition Department)
Expected results that can be leveraged
through publications in high-impact international journals, presentations at major cardiology and lipidology conferences, and the production of original data that can be reused in other cardiovascular research contexts.
Part of the project is supported by the Servier Institute and the French Society of Cardiology.







